- support
- info@evidentic.com
- +49 (0) 30 959 99 8831
1.962,00 €
Out of stock
*Subject to availability and market fluctuations.
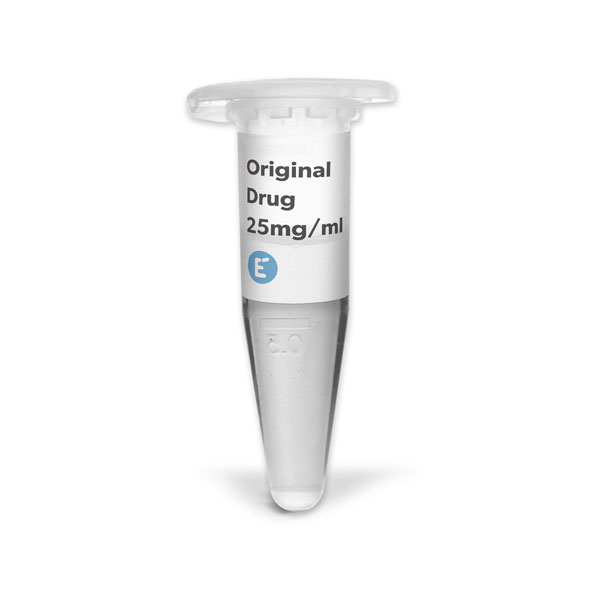
Evidentic offers repackaged finished pharmaceutical products (e.g RMP/RLD). We refer to the repackaged RMP/RLD products synonymously as drug aliquots or aRMP, or short: aliquots.
We offer batches of original drug aliquots in low-binding Eppendorf tubes for research use only. We provide licence-free drug aliquots with long, short or even “negative shelf life” by storing the products at recommended temperatures (either 2-8°C or -80°C) and ensuring fully functional molecules for research purposes.
Disodium phosphate dihydrate (E339)
Disodium edetate
Potassium chloride (E508)
Potassium dihydrogen phosphate (E340)
Polysorbate 80 (E433)
Sodium chloride
Evidentic GmbH
Martin-Buber-Str. 10
14163 Berlin