- support
- info@evidentic.com
- +49 (0) 30 959 99 8831
147,60 €
In stock
Our products are for research use only. The “therapeutic expiration date” implies expiry date for patient adminstration and is not applicable to the shelf life of the aliquot. Aliquot storage at -86°C ensures fully functional molecules even after the mentioned expiry date.
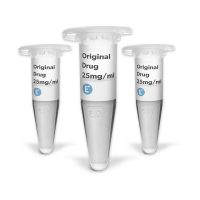
Evidentic offers repackaged finished pharmaceutical products (e.g RMP/RLD). We refer to the repackaged RMP/RLD products synonymously as drug aliquots or aRMP, or short: aliquots.
We offer batches of original drug aliquots in low-binding Eppendorf tubes for research use only. We provide license-free drug aliquots with long, short or even “negative shelf life” by storing the products at recommended temperatures (either 2-8°C or -80°C) and ensuring fully functional molecules for research purposes.
L-histidine hydrochloride monohydrate
L-histidine
α,α-trehalose dihydrate
Polysorbate 20

ProductBatch | Antigen | Molecular Class | Drug Brand | Product Concentration | CoA | Quantity per vial | Storage Temperature | Expiry Date | Price from | |
|---|---|---|---|---|---|---|---|---|---|---|
HER2 |
Biosimilar , Monoclonal Antibody |
Herzuma® |
21 mg/mL |
– |
6 mg |
tba |
tba |
356,00 € |
||
HER2 |
Biosimilar , Monoclonal Antibody |
Ontruzant® |
21 mg/mL |
– |
2 mg |
tba |
tba |
159,00 € |
||
HER2 |
Biosimilar , Monoclonal Antibody |
Kanjinti® |
21 mg/mL |
– |
6 mg |
-80°C |
01/2025 |
356,00 € |
||
HER2 |
Biosimilar , Monoclonal Antibody |
Zercepac® |
21 mg/mL |
– |
6 mg |
-80°C |
08/2026 |
356,00 € |
||
HER2 |
Biosimilar , Monoclonal Antibody |
Ogivri® |
21 mg/mL |
– |
6 mg |
-80°C |
10/2025 |
356,00 € |
||
HER2 |
Biosimilar , Monoclonal Antibody |
Trazimera® |
21 mg/mL |
– |
2 mg |
-80 °C |
09/2026 |
159,00 € |
||
HER2 |
Monoclonal Antibody |
Herceptin® |
21 mg/mL |
– |
2 mg |
-80°C |
08/2020 |
147,60 € |
||
HER2 |
Monoclonal Antibody |
Herceptin® |
21 mg/mL |
– |
2 mg |
-80°C |
02/2021 |
147,60 € |
||
HER2 |
Monoclonal Antibody |
Herceptin® |
21 mg/mL |
– |
2 mg |
-80°C |
08/2021 |
147,60 € |
||
HER2 |
Monoclonal Antibody |
Herceptin® |
21 mg/mL |
– |
2 mg |
-80°C |
11/2019 |
147,60 € |
||
HER2 |
Monoclonal Antibody |
Herceptin® |
21 mg/mL |
– |
2 mg |
-80°C |
03/2023 |
147,60 € |

Find and order your therapeutic molecules through smart filters
Up to 10 batches of original drugs for examining batch-to-batch variations

Up to 80% less compared to the original pharmaceutical price

Shipment within days worldwide according to GDP-standards

Up to 80% less compared to the original pharmaceutical price
Evidentic GmbH
Martin-Buber-Str. 10
14163 Berlin