- support
- info@evidentic.com
- +49 (0) 30 959 99 8831








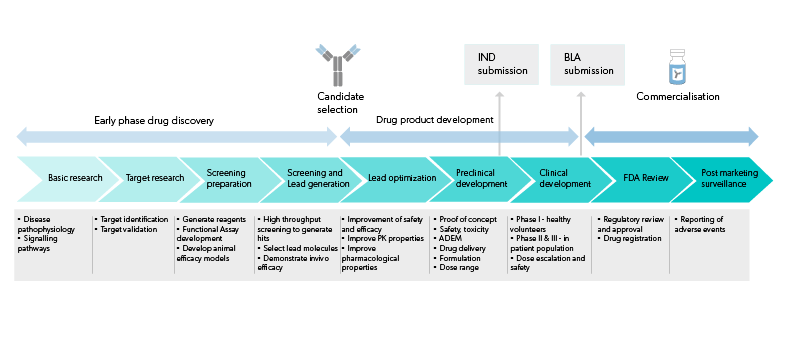
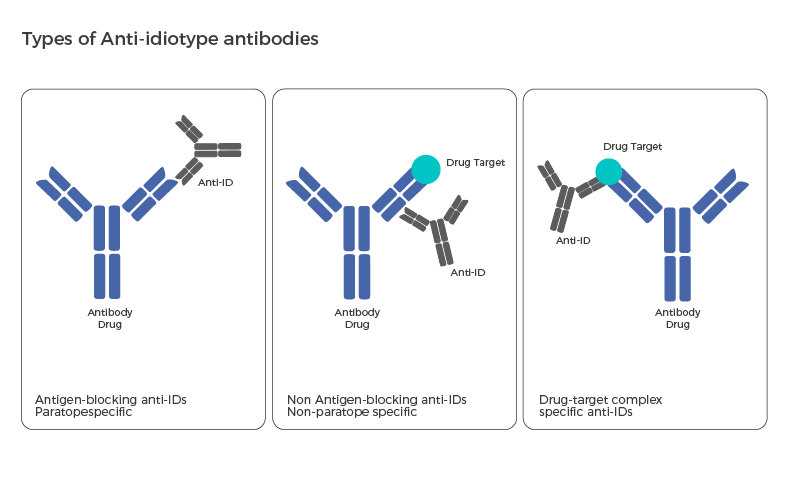
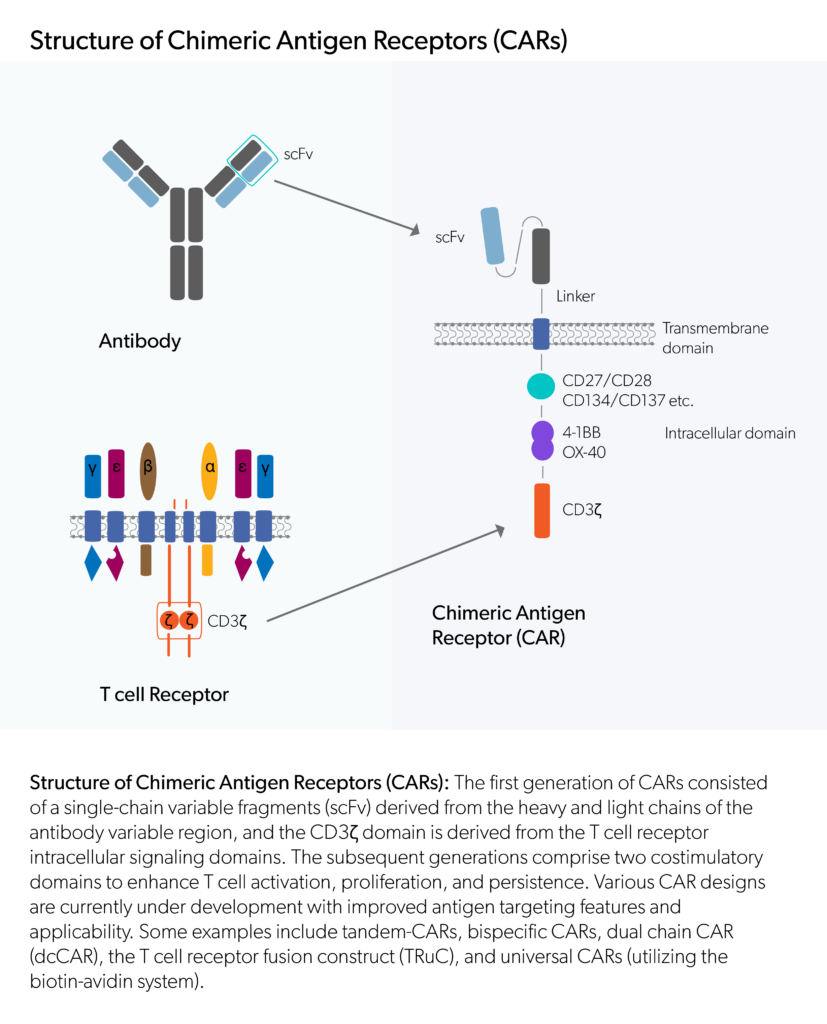
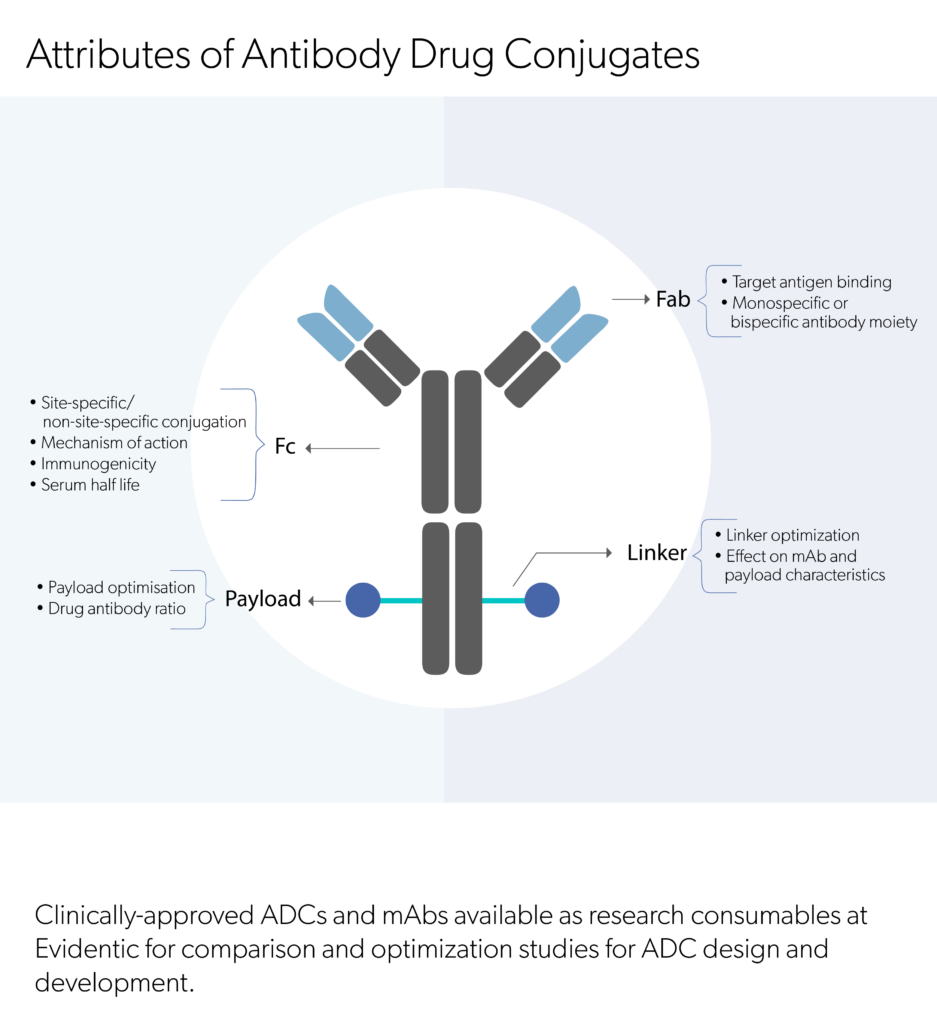
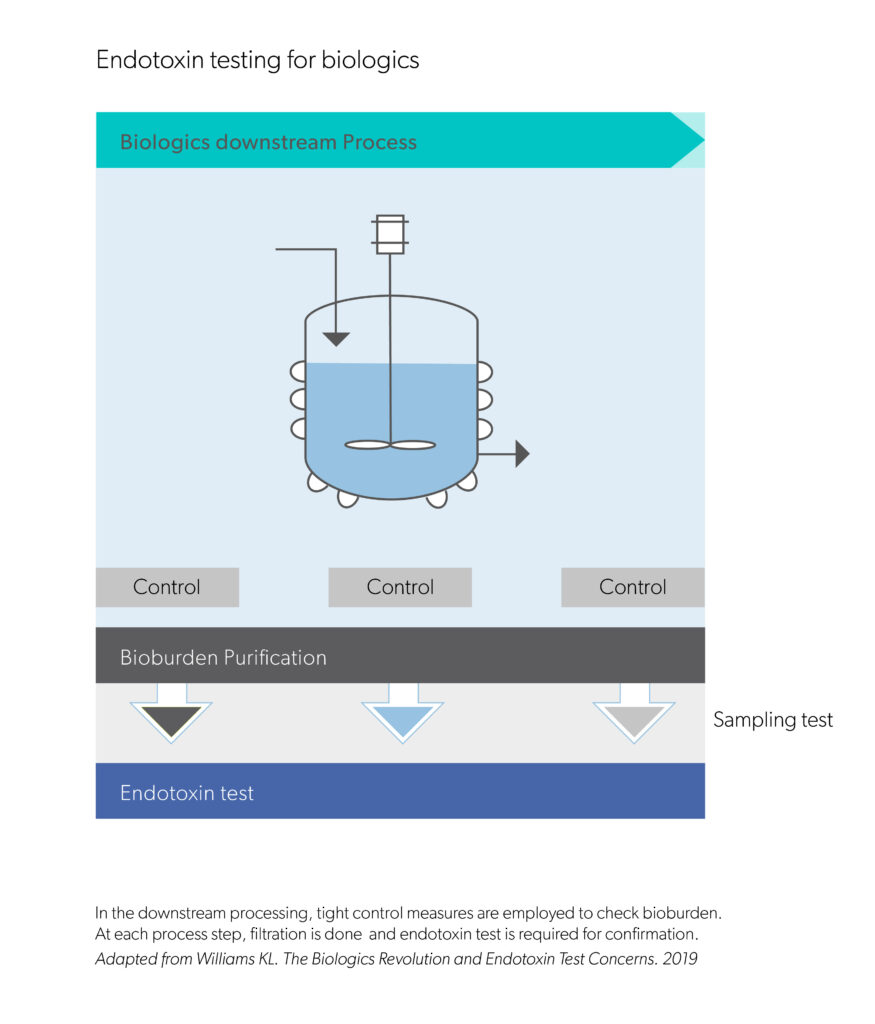
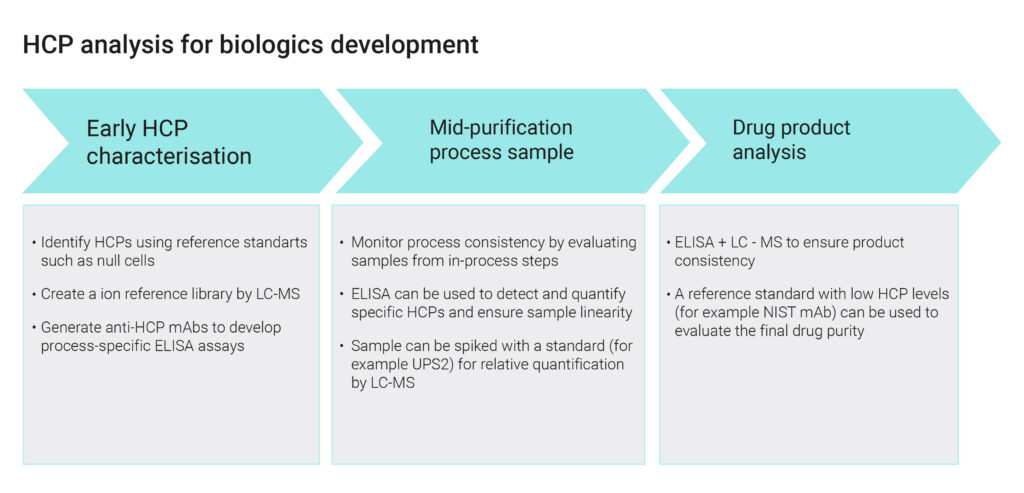
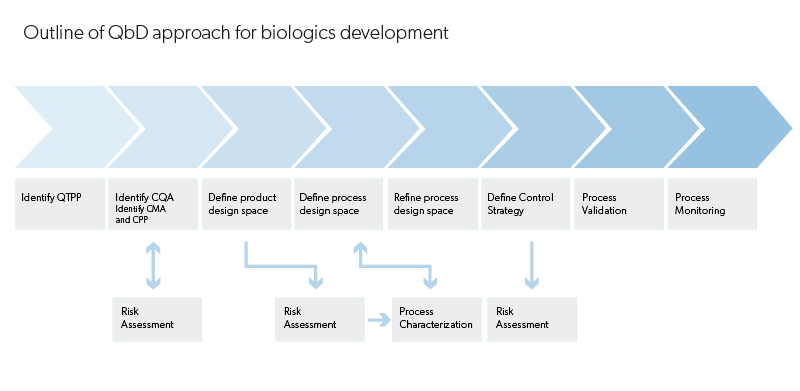
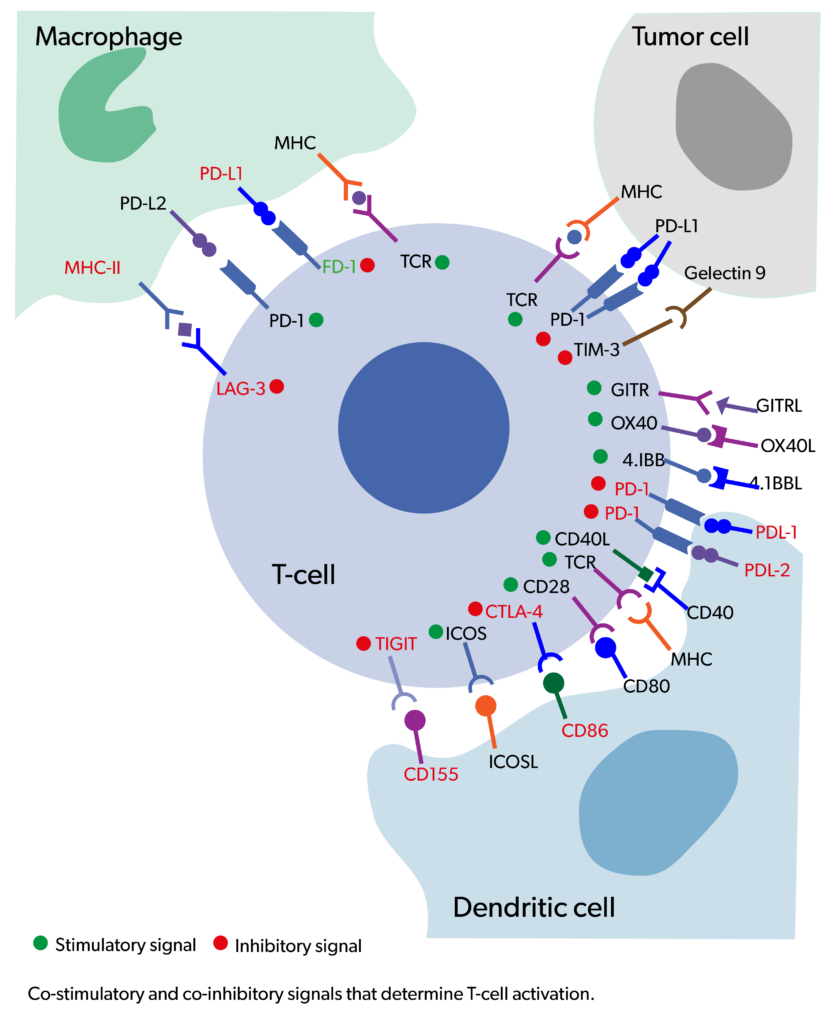
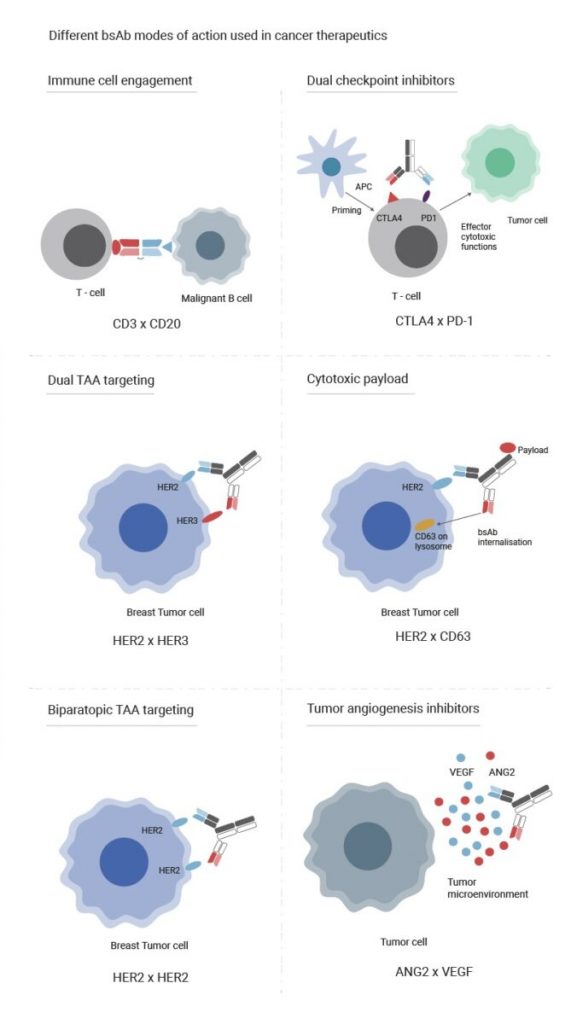

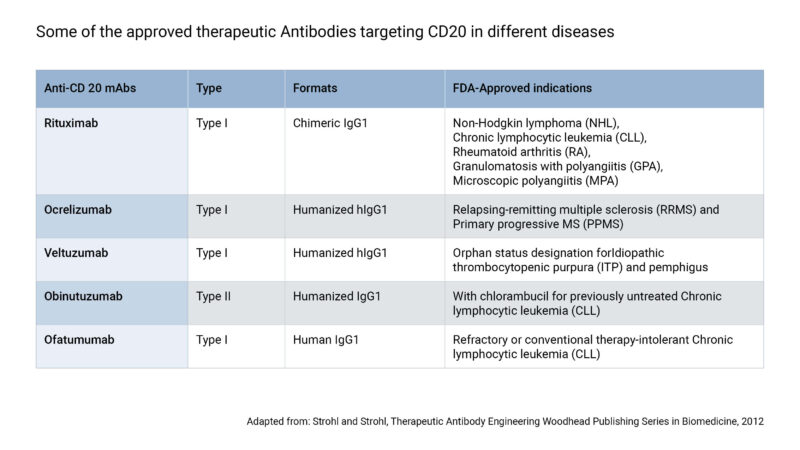
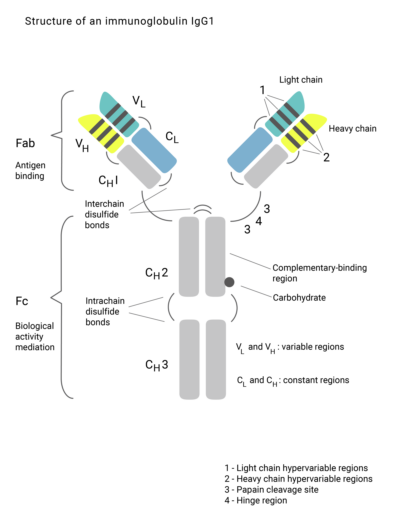
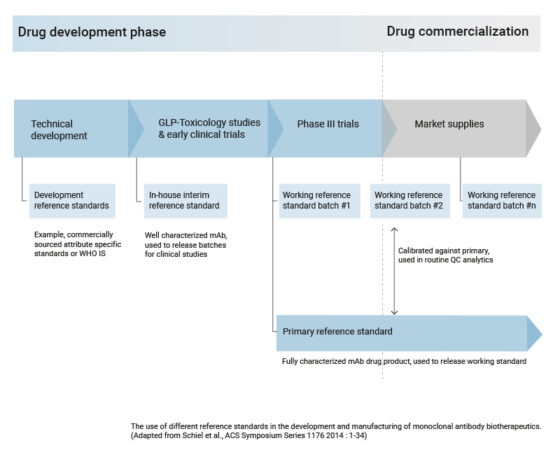






Evidentic GmbH
Martin-Buber-Str. 10
14163 Berlin