Monoclonal antibodies (mAbs) and related products are one of the fastest-growing classes of therapeutics. More than 80 molecules are presently approved and several 100s are under clinical development spanning a wide array of therapeutic areas (See article: Applications of mAbs). The recent advancements in antibody engineering technologies have enabled numerous antibody formats with distinct functional features. The degree of variability and complexity in structures call for extensive and comprehensive characterization studies in order to ensure safety and efficacy for human use.
Compared to small-molecule drugs, structural and functional characterization of mAb biologics can be a tedious and challenging process. It involves the use of state-of-the-art technologies to evaluate purity, specificity, potency, and toxicity, all in accordance with international regulatory guidelines. Characterization of therapeutic monoclonal antibodies is performed during both upstream and downstream processes in novel drug and biosimilar development. During early drug development, characterization studies are important to screen the right mAb molecule by defining the critical quality attributes (CQAs).
The CQAs encompass the physical, chemical, and biological properties of the molecule, which should be within a particular range to ascertain the product quality and clinical efficacy. As for process development and optimization of manufacturing processes, characterization studies are essential to identify the process-related impurities and for quality control. Moreover, the characterization of therapeutic monoclonal antibodies is a decisive step in the development of biosimilars. Comparative characterization studies with reference drugs are imperative for biosimilar approval.
Structural characterization of monoclonal antibodies
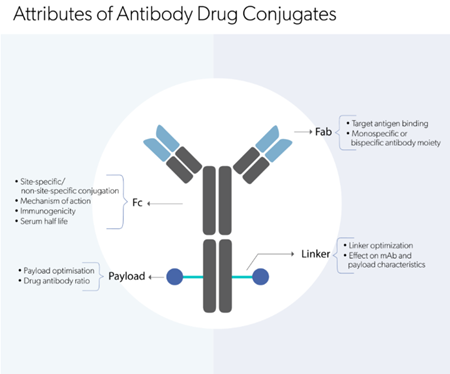
Most therapeutic antibodies are derivatives of IgG1 owing to the longer half-life, potent effector functional and relative structural homogeneity (Figure 1). However, their production by recombinant technology in live cells does often result in product heterogeneities. Subsequent exposure to culture media and storage conditions can add to the heterogeneity. Therefore, various physico-chemical assays are utilized to analyse the following mAb attributes.
Primary structure and Peptide mapping of monoclonal antibodies
- Delineating the DNA and amino acid sequence is the primary step in mAb characterization. Primary structure and molecular mass are usually analysed using mass spectrometry (MS) and reverse-phase high-performance liquid chromatography. In addition, genetic integrity and lot-to-lot variation can be assessed by peptide mapping of enzyme digested protein. Other analytical methods that are approved by regulatory agencies include capillary electrophoresis (CE) and the more robust sheathless interface-based transient isotachophoresis CE-ESI-MS.
Secondary and higher-order structures
- The secondary and tertiary structures directly translate to the functional activity of the protein and hence is a very important step in the characterization of monoclonal antibodies. The proportion of α-helix, β-sheet, and random coils are generally determined by Fourier to transform infrared spectroscopy (FT-IR) and circular dichroism (CD). Another important objective is to look into the disulfide bonds which give structural and functional stability to the molecular structure. This is usually achieved by collision-induced dissociation (CID) or electron-transfer dissociation (ETD), followed by MS. The tertiary structures are examined using highly sensitive spectroscopy methods, for example, ion-mobility MS and hydrogen-deuterium exchange MS.
Post-translational modifications (PTMs)
- The extent of PTMs can be partly predicted during peptide mapping. PTMs are classified into enzymatic and chemical modifications. Enzymatic modifications occur due to enzymatic catalysis after protein translation and include glycosylation, disulphide bond formation, and proteolytic cleavage. On the other hand, chemical modifications such as oxidation, de-amidation and glycation, can be a result of the upstream and downstream processes, formulation or storage. PTMs can have a profound impact on clinical performance, therefore must be well characterized and monitored during product development.
Aggregation
- Antibody dimerization and aggregation can alter immunogenicity which can have a direct effect on biological function. In addition, protein aggregation can pose difficulties during the downstream processes. Hence detection and characterization of aggregates become extremely important. Some of the most commonly used methods include high-performance size-exclusion chromatography, analytical ultracentrifugation, dynamic light scattering, differential scanning calorimetry and asymmetric flow field-flow fractionation.
Process-induced heterogeneity
- Certain analytical procedures and downstream processes can induce heterogeneity in molecular structures including charge, size and PTMs. Charge variants must be assessed in native proteins by solution-based methods such as CE-based isoelectric focusing or capillary isoelectric focusing (CIEF). Size variations are often a result of enzymatic or non-enzymatic cleavage, which are evaluated commonly using SDS-PAGE, size-exclusion chromatography, dynamic light scattering, and analytical ultracentrifugation and field-flow fractionation. PTMs such as N-linked glycosylation are very common in mAb products and can influence the effector functions. Therefore, methods such as MALDI-TOF and CE are frequently employed to detect and monitor glycosylation.

Functional characterization of monoclonal antibodies
Evaluation of the functional attributes is critical to determine the safety, toxicity and efficacy of mAb products. A systematic analysis of these attributes during pre-clinical in-vitro studies and animal studies is conducted before the candidate enters clinical trials. Functional characterization must be conducted during the early drug development phase, product characterization for lot release, and post-licensure life-cycle management.
Ligand binding studies
- The antibody-antigen interaction is the primary quality attribute that is analyzed. It is measured by the equilibrium dissociation constant (KD value) under various conditions, preferably by in-vitro methods such as SPR-based technology, fluorescence ELISA and kinetic exclusion assays (KinExA). Moreover, hydrogen-deuterium exchange mass spectrometry is often employed to determine the critical epitopes that are recognized by the mAb.
Cell-based assays
- Cell-based assays are very important to understand the mechanism of action (MoA) and evaluate the product potency. These assays are designed to evaluate the downstream cell-signalling events after ligand-binding. The biomarkers for downstream events must be well-defined (for example, inhibition of growth factors or induction of apoptotic factors) that are analysed using various qualitative and quantitative bioassays. These assays also give an idea of the impact of structural/chemical modifications on biological activity.
Assessment of effector functions for characterization of monoclonal antibodies
- The ligand-binding and MoA is usually determined by the Fab region of the mAb. However, the Fc region also plays an important role by influencing the serum half-life and effector functions such as antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cell-mediated phagocytosis (ADCP). Functional assays to determine effector functions are critical for the development of original drugs and biosimilars. Various bioassays using cell lines, for example, CHO M7 cells, baby rabbit complement, and macrophage-derived from monocytes are used to evaluate the levels of ADCC, CDC and ADCP respectively.
Speed up mAb characterization with Evidentic!

The molecular complexity and intrinsic heterogeneity of therapeutic monoclonal antibodies makes their development a challenging process. Extensive characterization becomes necessary at each stage of product development and manufacturing to ensure product efficacy and integrity. Although the first generation of biologics focused mainly on the ligand binding, the subsequent generations emphasize on the antigen affinity, valency, epitope and effector functions. This has also brought about an evolution of antibody formats that can specifically cater to different clinical outcomes. Thus, detailed characterization of molecular, biophysical, biochemical, biological, immunochemical, and immunological properties must be conducted using reliable reference products.
Evidentic offers a wide array of clinical-grade approved mAbs and other biologics which can be used as reference drugs for the development of innovative antibody molecules and biosimilars. Browse our portfolio for your desired molecule. We procure any EU-licenced molecule.
Discover clinical-grade molecules
References
- Wang X, An Z, Luo W, Xia N, Zhao Q. Molecular and functional analysis of monoclonal antibodies in support of biologics development. Protein Cell. 2018;9(1):74-85. doi:10.1007/s13238-017-0447-x
- Woojeong Kim W, Kui Hyun Kang KH and Jung-Keun Suh JK. Characterization of Biopharmaceuticals Focusing on Antibody Therapeutics. Biopharmaceuticals, IntechOpen, Nov 5, 2018. DOI: 10.5772/intechopen.79107
- Goulet DR, Atkins WM. Considerations for the Design of Antibody-Based Therapeutics. J Pharm Sci. 2020;109(1):74-103. doi:10.1016/j.xphs.2019.05.031